Corneal Health Solutions
Advancing the field of corneal remodeling
We’re transforming the standard of care for progressive keratoconus through our commitment to addressing important unmet clinical needs in corneal health.
With inspired innovation, a customer-centric focus, and prolific market access capabilities, we are in the constant pursuit of developing eye care solutions that empower ophthalmologists to deliver optimal care for patients with corneal disease.
Our proprietary corneal remodeling platform strengthens, stabilizes, and reshapes the cornea utilizing corneal cross-linking in minimally invasive and non-invasive outpatient procedures to treat corneal ectatic disorders and correct refractive conditions.
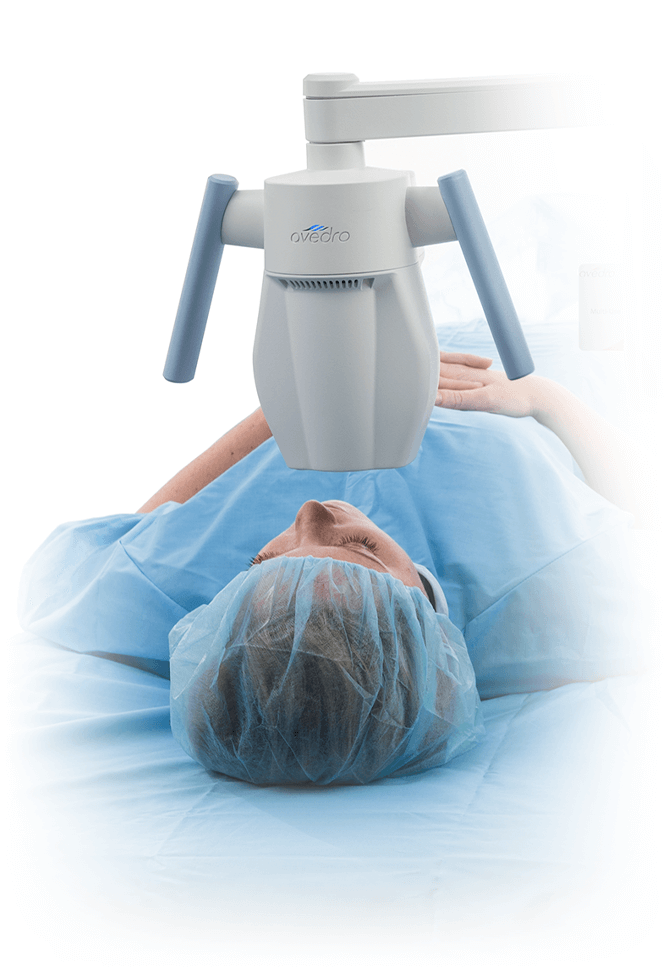
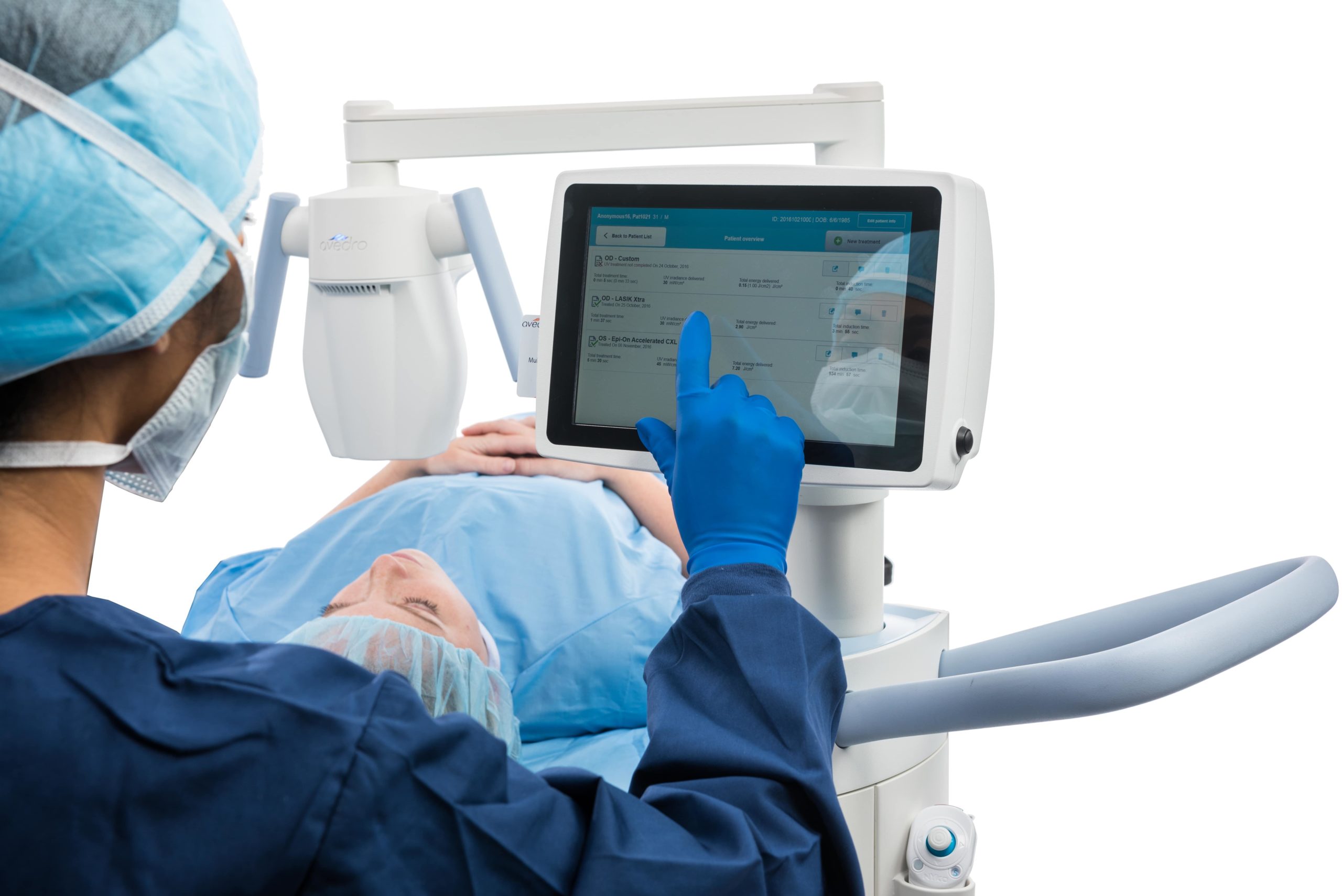
The KXL® system for performing corneal cross-linking and Lasik Xtra®

With treatment presets for all your cross-linking needs, including epi-off, epi-on, accelerated, pulsed or continuous treatment, and an on-board patient database, the KXL® system is designed for convenience and efficiency.
Learn More
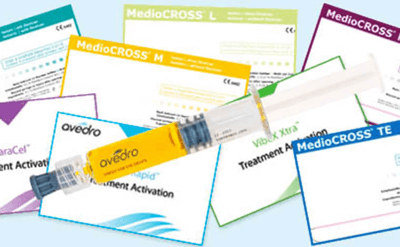
High-quality CE-marked riboflavin
Specifically designed formulations

Our scientifically developed family of CE-marked, quality riboflavin products ensure rapid diffusion in individual, sterile syringes for all of your cross-linking needs.
Learn More
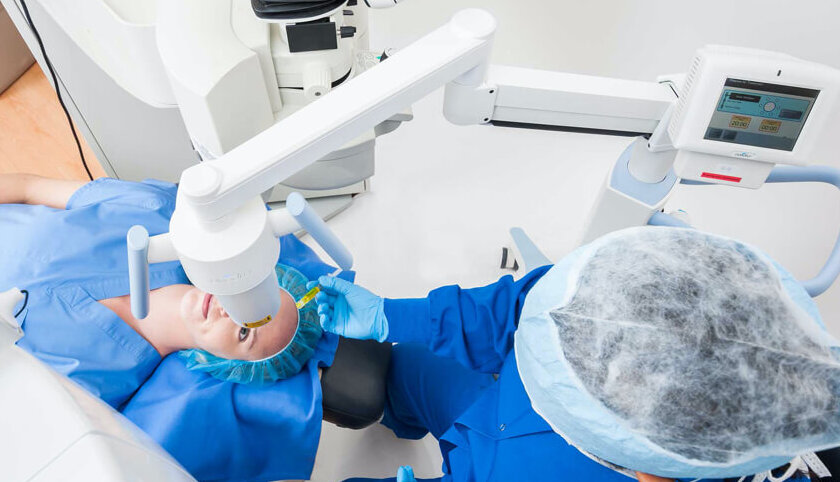
Enhancing Lasik

Performed in conjunction with LASIK, the Lasik Xtra procedure enhances the corneal biomechanical integrity.
Learn More
We want to hear from you!
Please contact us to learn more about our cross-linking products and procedures.
Contact Us
"*" indicates required fields
